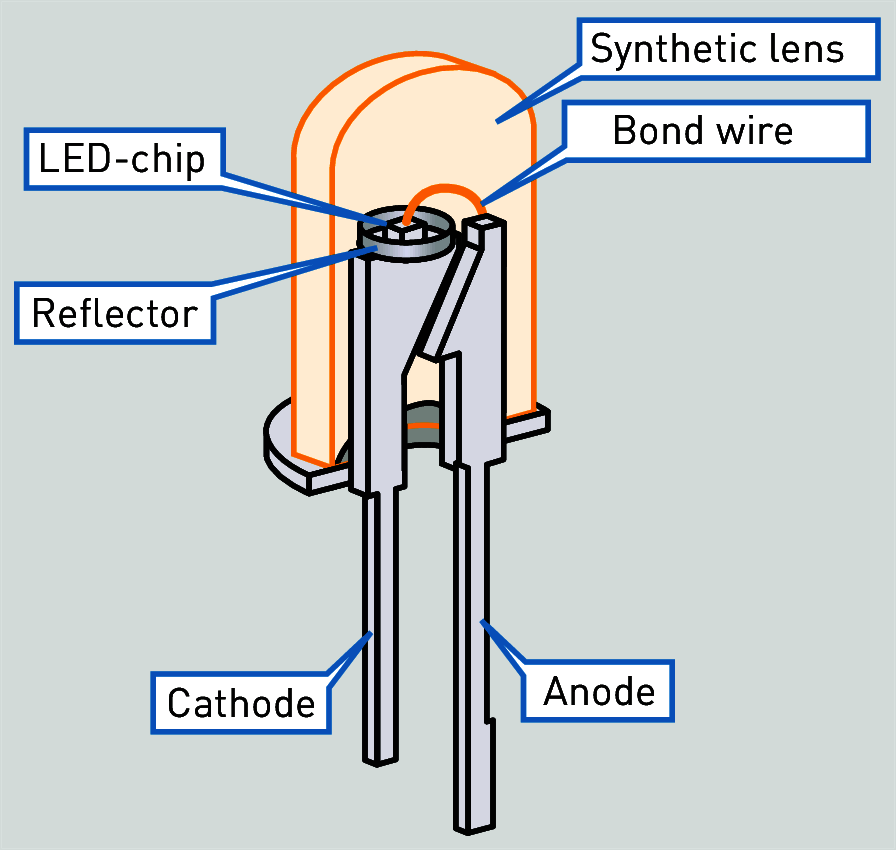
An LED, which stands for light-emitting diode, is a semiconductor diode that glows when a voltage is applied. Devices with LEDS are everywhere in our house: televisions, mobile phones, solar flood lights, torchlights, inhouse lighting, street lightings, and cars day- indicator lights. .
Many companies are producing LED lights, and there are ample choices to buy indoor lights, which previously were made by Philips and Osram using the older fluorescent lights. .
The working of an LED
In my MIT Sloan days, a well-known Professor of Innovation, James M. Utterback, brought to the class a collection of hairs (animal and human) that were used by Thomas Edison to develop his filament bulbs. Since then, filament bulbs have been used to light homes. Sadly, these filament bulbs would be largely substituted by LED lights of various shapes and sizes.
The workings of the LED bulb are vastly different from that of the older incandescent lightbulb. The incandescent light bulb works by running electricity through a filament that is inside the glass bulb. The filament heats up and glows, and that creates the light. However, it also creates a lot of heat. The incandescent light bulb loses about 98% of its energy-producing heat, making it quite inefficient.0datret light.
LED was built from a series of inventions
LEDs are part of a new family of lighting technologies called solid-state lighting; LEDs are cool to the touch. Instead of one lightbulb, in an LED lamp there are many small light-emitting diodes.
LEDs create light by electroluminescence in a semiconductor material. Electroluminance is a phenomenon of a material emitting light when electric current or an electric field is passed through it. This happens when electrons are sent through the materiel and fill electron holes. An electron hole exists where an atom lacks electrons (negatively charged) and therefore has a positive charge. Semiconductor materials like germanium or silicon can be “doped” to create and control the number of electron holes. Doping is the adding of other elements to the semiconductor material to change its properties. By doping a semiconductor, we can make two separate types of semiconductors in the same crystal. The boundary between the two types is called a p-n junction. The junction only allows current to pass through it one way. This is why they are used as diodes. LEDs are made using p-n junctions, As electrons pass through one crystal to the other they fill electron holes. They emit photons (light). This a complex process.
The pioneers of the LED
Currently the LED light is popular due to its efficiency and many believe it is a “new technology”. The LED, as we know it, has been around for over 50 years. The recent development of white LED is what has brought it into the public attention as a replacement for other white light sources.
The current state of the development of the LED was built on a series of innovations and their innovators. Many innovators have helped create the LED lights that are used today.
These innovators include:
- Henry Joseph Round
Electro luminesce, the natural phenomenon upon which LED technology is built, was discovered in 1907 by a British radio research and assistant to Guglielmo Marconi, Henry Joseph Round, while experimenting with silicon carbide and a cat’s whisker.
- Oleg Vladimirovich
During the 1920s, Russian radio researcher Oleg Vladimirovich was studying the phenomena of electroluminescence in diodes in radio sets. In 1927, he published a paper “Luminous Carborundum (silicon carbide) Detector and Detection with Crystals” detailing his research. While no practical LED was created at that time based on his work, his research did influence future inventors
- Robert Biard and Gary Pittman
Years later in 1961, Robert Biard and Gary Pittman invented and patented an infrared LED for Texas Instruments. This was the first LED. However, since it was infrared, it was beyond the visible light spectrum. Human cannot see infrared light. Biard only accidentally invented a light-emitting diode while they were actually attempting to invent a laser diode.
- Nick Holonyack
In 1962, Nick Holonyack, a consulting engineer for General Electric, invented the first visible light LED. It was a red LED and Holonyack used gallium arsenide phosphide as a substrate for the diode. Holonyack has earned the honour of being called the “Father of light -emitting diode” for his contribution. .
He also holds 41 patents and his other inventions include the laser diode and the first light dimmer.
- M. George Craford
In 1972, electrical engineer, M. George Craford, invented the first yellow-coloured LED for Monsanto using gallium arsenide phosphide in the diode. Craford also invented a red LED that was 10 times brighter than Holonyack’s.
Monsanto was the first company to mass-produce visible LEDs. In 1968. Monsanto produced red LEDs used as indicators. But it was not until the 1970s that LED became popular when n Fairchild Optoelectronics began producing low-cost LED devices for manufacturers.
- Herbert Maruska and Waalden C. Rhines.
In 1986, Herbert Maruska and Walden C. Rhines from Stanford University, US, created a working blue LED using magnesium, and set all future standards.
- Isamu Araski and Hiroshi Amano
In 1993, physicists Isamu Araski and Hiroshi Amano developed a high-quality gallium nitride for blue LEDs.
- Shuji Nakamura
In 1979, Shuji Nakamura developed the world’s first bright blue LED using gallium nitride. It wouldn’t until the 1990s that the blue LED would become low cost for commercial production. These developments led to the development of white LEDs.
The Importance of white light LEDs
Blue LEDs have been developed based on gallium nitride and silicon carbide materials. Production of light in this shorter-wavelength, more energetic region of the visible spectrum, has long been elusive to designers of LEDs. High photon energies (light) typically increase the failure rate of semiconductor devices, and the low sensitivity of the human eye to blue light adds to the brightness requirement for a useful blue diode. One of the most important aspects of a blue LED is that it completes the red, green, and blue (RGB) primary color family to provide an additional mechanism of producing solid-state white light, through the mixing of these component colors.
The addition of bright blue-emitting LED to the earlier-developed red and green devices makes it possible to use three LEDs, tuned up to an appropriate output levels, to produce any color of the visible light spectrum, including white. Other possible approaches to producing white light, utilizing a single device, are based on phosphor or dye wavelength converters or semiconductor wavelength converters. The concept of white LED is particularly attractive for general illumination, due to the reliability of solid-state devices, and the potential for delivering very high luminous efficiency as compared to conventional incandescent and fluorescent sources.
The human eye perceives light as being white if the three types of photosensory cone cells, located in the retina, are attenuated in particular ratios. The three cone types exhibit response curves that peak in sensitivity at wavelength representing red, green and blue, and the combination of these response signals produces various color sensations in the brain. A wide variety of different color mixtures are capable of producing a similar perceived color, especially in the case of white, which may be realized through many combinations of two or more colors.
A chromaticity diagram is a graphical means of representing the results obtained from mixing colors. Monochromatic color appear on the periphery of the diagram, and a range of mixture representing white is located in the central region of the diagram. Light that is perceived as white can be generated by different mechanisms. A chromaticity diagram is shown in Table 1.
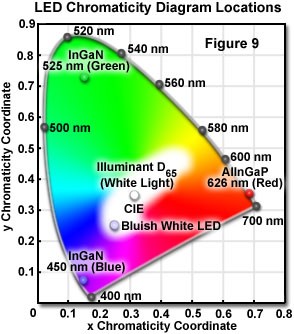
Table 1: LED chromaticity diagram
One method is to combine light of two complementary colors in the proper power ratio. The ratio that produces tristimulus response to the retina (causing perception of white) varies for different color combinations.
A selection of complementary wavelengths, along with the power ratio for each pair that produces the chromaticity coordinates of a standard illuminant is designated D(65) by the International Commission for Illumination (CIE, Commission Internationale de I’Eclairage).
Another means of generating white light is by combining the emission of three colors that will produce the perception of white light when they are combined in the proper power ratio. White light can also be produced by broadband emission from a substance that emits over a large region of the visible spectrum. This type of emission approximates sunlight, and is perceived as white. Additionally, broadband emission can be combined with emission at discrete spectral line to produce a perceived white, which may have particular desirable color characteristics that differ from these of white light produced by other techniques.
The combination of red, green, and blue diode chips into one discrete package, or in a lamp assembly housing a cluster of diodes, allows the generation of white light or any of 256 colors by utilizing circuitry that drives the three diodes independently. In applications requiring a full spectrum of colors from a single point source, this type of RGB diode format is the preferred technique.
Most white-light diodes employ a semiconductor chip emitting at a short wavelength (blue, violet or ultraviolet) and a wavelength converter, which absorbs light from the diode and undergoes secondary emission at a longer wavelength. Such diodes, therefore, emit light of two or more wavelengths, that when combined, appear as white. The quality and spectral characteristics of the combined emission vary with the different design variations that are possible. The most common wavelength converter materials are termed phosphors, which exhibit luminescence when they absorb energy from another radiation source. The typically utilized phosphors are composed of an inorganic host substance containing an optically active dopant. Yttrium aluminium garnet (YAG) is a common host material, and for diode applications, it is usually doped with one of the rare-earth elements or a rare-earth compound. Cerium is a common dopant element in YAG phosphors designed for white light emitting diodes.
The first commercially available white LED (fabricated and distributed by the Nichia Corporation) was based on a blue-light-emitting gallium-indium-nitride (GaInN) semiconductor device surrounded by a yellow phosphor. The phosphor is Ce-doped YAG, produced in powder form and suspended in the epoxy resin used to encapsulate the die. The phosphor-epoxy mixture fills the reflector cup that supports the die on the lead frame, and a portion of the blue emission from the chip is absorbed by the phosphor and reemitted at the longer phosphorescence wavelength. The combination of the yellow photo-excitation under blue illumination is ideal in that only one converter species is required. Complementary blue and yellow wavelengths combine through additive mixing to produce the desired white light. The resulting emission spectrum of the LED represents the combination of phosphor emission, with the blue emission that passes through the phosphor coating unabsorbed.
The relative contributions of the two emission bands can be modified to optimize the luminous efficiency of the LED, and the color characteristics of the total emission. These adjustments can be accomplished by changing the thickness of the phosphor-containing epoxy surrounding the die, or by varying the concentration of the phosphor suspended in the epoxy. The bluish white emission from the diode is synthesized, in effect, by additive color mixing, and its chromaticity characteristics are represented by a central location (0.25, 0.25) on the CIE chromaticity diagram ( Bluish White LED).
White light diodes can generate emission by another mechanism, utilizing broad-spectrum phosphors that are optically excited by ultraviolet radiation. In such devices, an ultraviolet-emitting diode is employed to transfer energy to the phosphor, and the entire visible emission is generated by the phosphor. Phosphors that emit at a broad range of wavelengths, producing white light, are readily available as the materials used in fluorescent light and cathode ray tube manufacture. Although fluorescent tubes derive their ultraviolet emission from a gas discharge process, the phosphor emission stage producing white light output is the same as in ultraviolet-pumped white diodes. The phosphors have well known color characteristics and diodes of this type have the advantage that they can be designed for applications requiring critical color rendering. A significant disadvantage of the ultraviolet-pumped diodes, however, is their lower luminous efficiency when compared to white diodes employing blue light for phosphor excitation. This results from the relatively high energy loss in the down-conversion of ultraviolet light to longer visible wavelengths.
Dyes are another suitable type of wavelength converter for white diode applications, and can be incorporated into the epoxy encapsulant or in transparent polymers. The commercially available dyes are generally organic compounds, which are chosen for a specific LED design by consideration of their absorption and emission spectra. The light generated by the diode must match the absorption profile of the converting dye, which in turn emits light at the desired longer wavelength. The quantum efficiencies of dyes can be near 100 percent, as in phosphor conversion, but they have the disadvantage of poorer long-term operational stability than phosphors. This is a serious drawback, as the molecular instability of the dyes causes them to lose optical activity after a finite number of absorptive transitions, and the resulting change in light emitting diode color will limit its lifetime.
White light LEDs based on semiconductor wavelength converters have been demonstrated that are similar in principle to the phosphor conversion types, but which employ a second semiconductor material that emits a different wavelength in response to the emission from the primary source wafer. These devices have been referred to as photon recycling semiconductors (or PRS-LEDs), and incorporate a blue-emitting LED die bonded to another die that responds to the blue light by emitting light of a complementary wavelength. The two wavelengths then combine to produce white. One possible structure for this type of device utilizes a GaInN diode as a current-injected active region coupled to an AlGaInP optically-excited active region. The blue light emitted by the primary source is partially absorbed by the secondary active region, and “recycled” as reemitted photons of lower energy. In order for the combined emission to produce white light, the intensity ratio of the two sources must have a specific value that can be calculated for the particular dichromatic components. The choice of materials and the thickness of the various layers in the structure can be modified to vary the color of the device output.
Because white light can be created by several different mechanisms, utilizing white LEDs in a particular application requires consideration of the suitability of the method employed to generate the light. Although the perceived color of light emitted by various techniques may be similar, its effect on color rendering, or the result of filtration of the light, for example, may be entirely different. White light created through broadband emission, through mixing of two complementary colors in a dichromatic source, or by mixing of three colors in a trichromatic source, can be located at different coordinates on the chromaticity diagram and have different color temperatures with respect to illuminants designated as standards by the CIE. It is important to realize, however, that even if different illuminants have identical chromaticity coordinates, they may still have substantially different color rendering properties, due to variations in details of each source’s output spectrum.
Two factors, referred to previously, are of primary importance in evaluating white light generated by LEDs: the luminous efficiency, and the color rendering capabilities. A property referred to as the color rendering index (CRI) is utilized in photometry to compare light sources, and is defined as the source’s color rendering ability with respect to that of a standard reference illumination source. It can be demonstrated that there exists a fundamental trade-off between luminous efficiency and color rendering ability of light-emitting devices. For an application such as signage, which utilize blocks of monochromatic light, the luminous efficiency is of primary importance, while the color rendering index is irrelevant. For general illumination, both factors must be optimized.
The spectral nature of the illumination emitted from a device has a profound influence on its color rendering ability. Although the highest possible luminous efficiency can be obtained by mixing two monochromatic complementary colors, such a dichromatic light source has a low color rendering index. In a practical sense, it is logical that if a red object is illuminated with a diode emitting white light created by combining only blue and yellow light, then the appearance of the red object will not be very pleasing. The same diode would be quite suitable for backlighting a clear or white panel, however. A broad-spectrum white light source that simulates the sun’s visible spectrum possesses the highest color rendering index, but does not have the luminous efficiency of a dichromatic emitter.
Phosphor-based LEDs, which either combine blue emission wavelengths with a longer-wavelength phosphorescence color, or create light solely from phosphor emission (as in ultraviolet-pumped LEDs), can be designed to have color rendering capabilities that are quite high. They have color character that is similar in many respects to that of fluorescent lamp tubes. The GaInN LEDs utilize blue emission from the semiconductor to excite phosphors, and are available in cool white, pale white, and incandescent white versions that incorporate different amounts of phosphor surrounding the chip. The cool white is the brightest, utilizing the least phosphor, and produces light with the most bluish color. The incandescent white version surrounds the blue-emitting chip with the most phosphor, has the dimmest output, and the yellowest (warmest) color. The pale white has brightness and color shade characteristics intermediate between the other two versions.
The availability of white LEDs has generated great interest in applying these devices to general lighting requirements. As lighting designers become familiar with the characteristics of the new devices, a number of misconceptions will have to be dispelled. One of these is that the light from a white LED can be used to illuminate a lens or filter of any color, and maintain the accuracy and saturation of the color.
In a number of the versions of white LED, there is no red component present in the white output, or there are other discontinuities in the spectrum. These LEDs cannot be used as general sources to backlight multicolored display panels or colored lenses, although they function well behind clear or white panels. If a blue-based GaInN white LED is employed behind a red lens, the light transmitted will be pink in color. Similarly, an orange lens or filter will appear yellow when illuminated with the same LED. Although the potential benefits in application of LEDs are tremendous, consideration of their unique characteristics is necessary in incorporating these devices into lighting schemes in place of more familiar conventional sources.
Conclusion
White LEDs are becoming popular sources of while lights. There were many inventors involved since the early 1900s to bring about this useful and practical white LEDs and its applications.
Let us salute those inventors who were from various nationalities.
References:
There are many sources of articles on LEDS on the internet. The most useful reference was from https://micromagnet.fsu.edu/primer/lightand color/ledintro.html.
